Mark Paddock, Ph.D University of California, San Diego
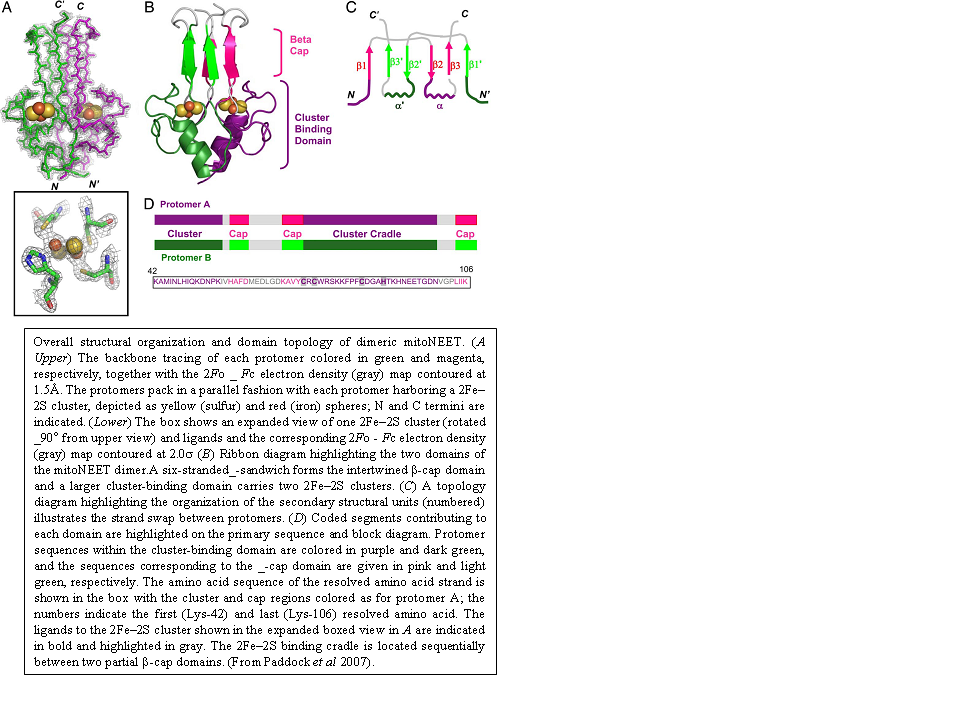
In an effort to understand the structural properties of the outer mitochondrial membrane protein mitoNEET, I crystallized a soluble form of the human protein in an orthorhombic space group. The structure was determined by X-ray diffraction from 1.5 Å resolution data collected from SSRL. To our surprise, the crystal structure showed that mitoNEET folds into a unique homodimeric structure with one 2Fe-2S cluster bound to each monomer within the dimer (see Figure below). A structural similarity search revealed that this fold is novel when compared with the > 700 known Fe-S proteins, and, furthermore, it is also unique when compared with the > 68,000 known members of the structural databases. In addition to the unique overall fold, the structure shows the unique 3Cys-1His coordination of the 2Fe-2S centers and an unusual distribution of hydrophobic and charged amino acid side chains.
The crystal structure of the human paralog Miner1 was also determined. Mis-splicing of CISD2, which codes for Miner1, is causative in Wolfram Syndrome 2 (WFS2) resulting in early onset optic atrophy, diabetes mellitus, deafness and decreased lifespan. In knock-out studies, disruption of CISD2 leads to accelerated aging, blindness and muscle atrophy. The soluble region of human Miner1, solved to a resolution of 2.1 Å (R-factor=17%), showed that it has a similar protein fold to mitoNEET with two redox-active 2Fe-2S centers, each bound by a rare 3Cys-1His motif. Miner1 is the first functionally different protein that shares the NEET fold with its recently identified paralog mitoNEET, an outer mitochondrial membrane protein.